Abstract

Introduction: Methotrexate (MTX) given on days 1, 3, 6 (and 11) after allogeneic haematopoietic cell transplantation (allo-HCT) along with calcineurin inhibitors (CNI) was established as effective GVHD prophylaxis decades ago when myeloablative conditioning was standard. Mycophenolate mofetil (MMF) replaced MTX initially in minimal intensity conditioning protocols and then became increasingly popular due to its favorable toxicity profile. Although MMF was associated with more severe GVHD in some reports, similar survival outcomes were reported for both regimens in most randomized trials. However, two large retrospective studies from the CIBMTR reported inferior outcomes of MMF-based regimens in allo-HCT standard indications. Taking advantage of a very large sample size within the EBMT registry, the aim of this retrospective study was to determine outcome differences after allo-HCT between MTX- and MMF-based GVHD prophylaxis regimens considering subgroup heterogeneity.
Patients and Methods: Eligible were patients from the EBMT registry with select chronic myeloid malignancies (CML, CMML, MDS, myelofibrosis, secondary AML) who were transplanted from matched related, matched unrelated and mismatched unrelated donors between 2007 and 2017 and received either MTX or MMF prophylaxis in combination with a CNI. Endpoints were overall (OS) and relapse-free survival (RFS), relapse incidence, non-relapse mortality (NRM), and cumulative incidence of grade 2-4 acute GVHD. Multivariable Cox regression analyses included age, conditioning intensity, TBI, diagnosis, disease stage, ATG, graft source, donor type, gender match, CMV (recipient), year of transplantation, and Karnofsky index. We tested whether the association between the type of prophylaxis and the outcome was different in patient subgroups by including interaction terms in multivariable analyses between prophylaxis type (MTX/MMF) and ATG, age, Tacrolimus, conditioning intensity, use of TBI, diagnosis, disease stage and recipient CMV status. Finally, we studied the association between the same set of variables and OS, relapse and NRM in patients after aGvHD and separately in a landmark analysis at 3 months in patients without aGvHD.
Results: Overall, 13699 patients from 321 centers were included. Diagnoses were MDS (44%), Myelofibrosis (18%), CML (11%), CMML (6%), atypical CML (1%) and secondary AML (21%). The conditioning / prophylaxis combination was RIC/MMF in 34%, RIC/MTX in 22%, MAC/MMF in 12% and MAC/MTX in 32%. CNI prophylaxis was Tacrolimus (n=1248), ciclosporin (n=12286), both (n=165). Median follow up was 42.8 months (IQR 19.8-74.5 months).
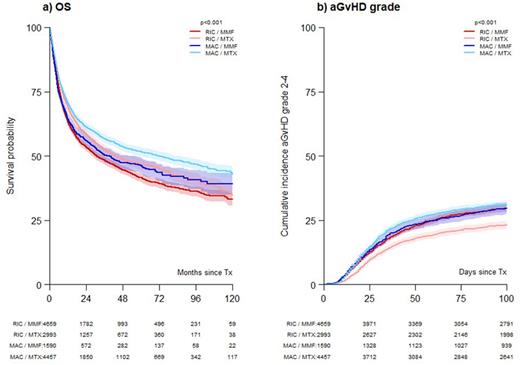
Unadjusted analyses demonstrated significantly better outcomes with MTX in all subgroups of patients (TBI/no TBI, RIC/MAC, diagnoses except myelofibrosis, ATG/no ATG, age at allo-HCT < or ≥60 years). Figure 1a shows OS curves for conditioning/prophylaxis combinations. The cumulative incidence of acute GVHD grade 2-4 was lower in in particular RIC patients (Figure 1b).
MTX was associated with significantly reduced overall mortality (HR 0.88, 95%CI 0.81-0.95, p<0.001) and NRM (HR 0.87, 95% CI 0.78-0.96, p=0.008) in multivariable analyses in the whole cohort. In contrast, MTX prophylaxis was not significantly associated with relapse (HR 1.02, 95% CI 0.92-1.12, p=0.72) and relapse-free survival (RFS) (HR 0.94, 95% CI 0.88-1.01, p=0.11).
Notably, there was no significant interaction effect between prophylaxis type and any of the eight variables (ATG, age, tacrolimus, conditioning intensity, use of TBI, diagnosis, disease stage and CMV status in recipients) tested for OS and NRM, except that the beneficial association of MTX on OS was weaker for patients with Myelofibrosis.
OS after acute GVHD was significantly lower with MTX prophylaxis and this was similar in RIC and MAC. MTX did not significantly associate with improved outcome in a landmark analysis in patients without aGvHD at 3 months after allo-HCT.
Conclusion: Compared to MMF, MTX-complemented calcineurin inhibitor prophylaxis was associated with favorable early OS and NRM, and in particular with favorable OS after aGVHD, in this large cohort of patients with chronic myeloid malignancies or sAML transplanted in a recent time period. This effect was observed across various subgroups. These results may have future impact on clinical practice and trial design.
Disclosures
Luft:JAZZ Pharmaceuticals: Consultancy. Kröger:Takeda: Consultancy, Honoraria; Sanofi: Honoraria; Kite: Honoraria; Neovii: Honoraria, Research Funding; Riemser: Research Funding; DKMS: Research Funding; Amgen: Honoraria; BMS: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Jazz: Honoraria. Platzbecker:Janssen: Honoraria; Jazz: Honoraria; Silence Therapeutics: Honoraria; Takeda: Honoraria; Novartis: Honoraria; Abbvie: Honoraria; BMS/Celgene: Honoraria; Geron: Honoraria. Schetelig:Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees. Dreger:AbbVie, AstraZeneca, Gilead, Novartis, Riemser, and Roche: Speakers Bureau; AbbVie, AstraZeneca, Bluebird Bio, Gilead, Janssen, Novartis, Riemser, and Roche: Consultancy. Yakoub-Agha:Kite, a Gilead Company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support; Bristol Myers Squibb: Honoraria; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria. Mielke:BMS/Celgene: Speakers Bureau; Novartis: Speakers Bureau; Kite/Gilead: Other: Expert Panel w/ Travel costs ; Miltenyi: Other: DSMB. McLornan:NOVARTIS: Honoraria, Research Funding, Speakers Bureau; JAZZ: Honoraria, Speakers Bureau; ABBVIE: Speakers Bureau; CELGENE BMS: Research Funding, Speakers Bureau. Robin:MEDAC, ASTEX: Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal